Lithium-ion batteries power countless devices in modern life, from smartphones and laptops to electric vehicles and e-bikes. UK fire services now tackle at least three lithium-ion battery fires every day, with incidents increasing by 46% in 2023 alone. These fires pose unique dangers because they burn extremely hot, release toxic gases, and can reignite even after appearing extinguished.
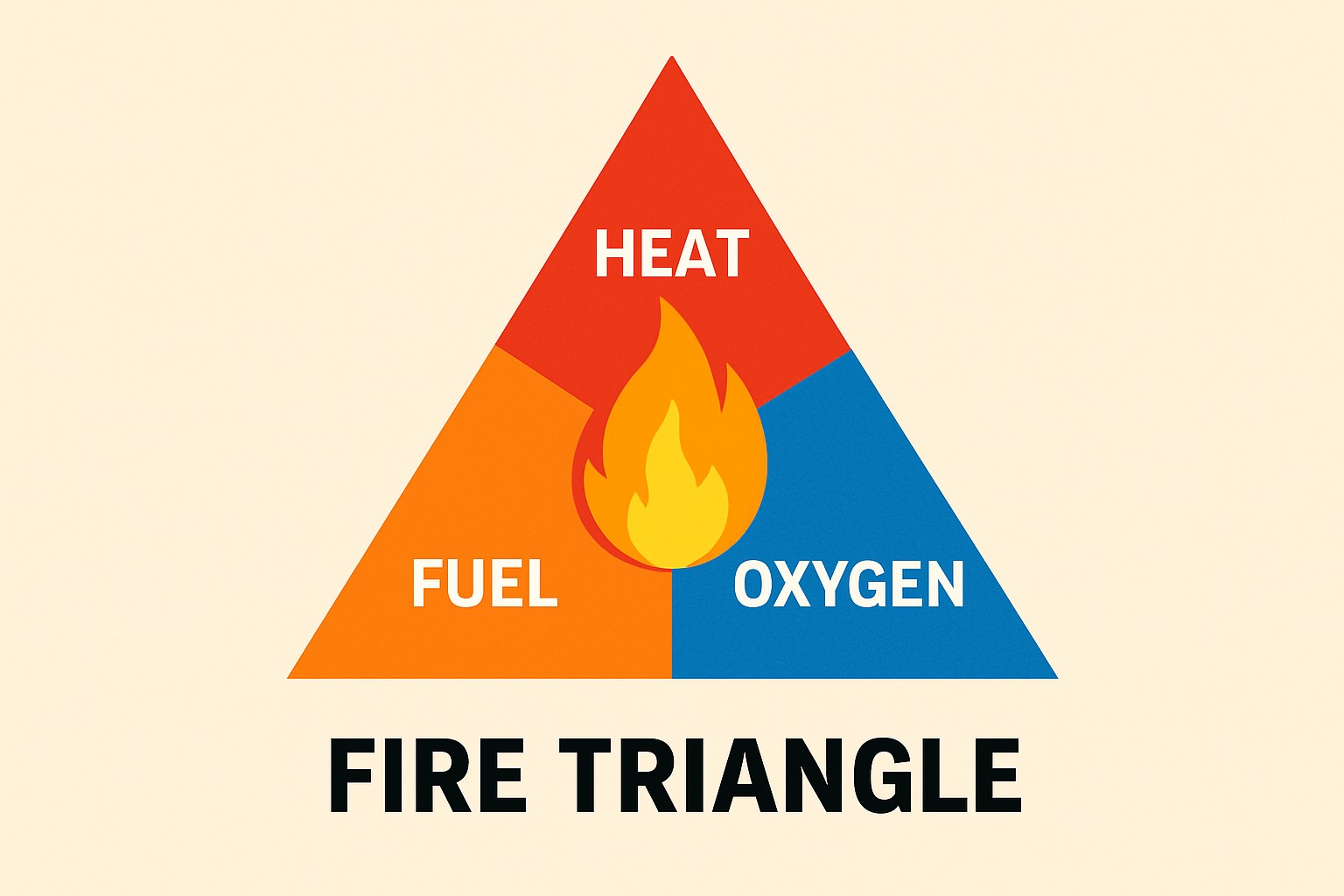
Understanding why these batteries catch fire and how to prevent incidents can protect lives and property. The causes range from manufacturing defects and physical damage to improper charging and storage conditions. When lithium batteries fail, they enter a process called thermal runaway, which creates intense heat and spreads rapidly to nearby cells.
Battery fires can be particularly challenging to control compared to traditional fires. Learning proper safety measures, storage techniques, and emergency responses helps reduce the growing risk these essential power sources present in homes, workplaces, and transport systems.
Key Takeaways
- Lithium battery fires occur when damaged or defective batteries overheat and enter thermal runaway
- Proper charging, storage, and handling practices significantly reduce fire risks
- These fires require special extinguishing methods and can reignite hours after being put out
Looking for a fire marshal qualification? We offer a range of online RoSPA approve courses.
Understanding Lithium-Ion Batteries
Lithium-ion batteries work through the movement of lithium ions between electrodes during charging and discharging. These rechargeable power sources have become essential in countless devices from mobile phones to electric vehicles.
Key Components and Operation
Lithium-ion batteries contain four main parts that work together to store and release energy. The cathode serves as the positive electrode and typically contains lithium compounds like lithium cobalt oxide or lithium iron phosphate.
The anode acts as the negative electrode and is usually made from graphite. Between these electrodes sits an electrolyte solution that allows lithium ions to move back and forth.
A separator keeps the cathode and anode apart whilst letting ions pass through. This thin barrier prevents dangerous short circuits that can lead to thermal runaway reactions.
During charging, lithium ions move from the cathode to the anode through the electrolyte. When the battery powers a device, the ions flow back to the cathode, creating an electric current.
This process repeats hundreds or thousands of times during the battery's lifetime. However, damage to any component can disrupt normal operation and create fire risks.
Widespread Applications and Usage
Modern life depends heavily on lithium-ion batteries across multiple sectors. Smartphones, laptops, electric vehicles, power tools, and other portable electronics all rely on this technology for reliable power storage.
Electric bikes and scooters use larger lithium-ion battery packs for transportation. Many homes now have backup power systems and solar energy storage that use these batteries.
Commercial buildings often install large-scale lithium-ion systems for energy management. Emergency lighting, medical devices, and security systems also depend on these batteries.
The popularity stems from their ability to store a high amount of energy in compact, lightweight packages. They charge faster than older battery types and hold their charge longer when not in use.
However, this widespread adoption means more potential fire risks in homes, workplaces, and public spaces.
What Is a Lithium Battery Fire?
Lithium battery fires are intense blazes caused by thermal runaway in lithium-ion cells. These fires produce toxic gases, generate their own oxygen, and resist traditional extinguishing methods.
Definition and Unique Characteristics
A lithium-ion battery fire occurs when the battery cell overheats and experiences thermal runaway. This creates a chain reaction where the battery produces more heat than it can release.
Thermal runaway happens when lithium-ion batteries overheat due to damage, overcharging, or manufacturing defects. The battery's electrolyte breaks down into flammable and toxic gases.
Key characteristics of lithium-ion battery fires include:
- Self-sustaining combustion - The battery generates its own oxygen during thermal runaway
- Toxic gas emission - Hydrogen fluoride and other dangerous chemicals are released
- Extreme temperatures - Fires can reach over 500°C
- Reignition risk - Batteries can restart burning hours or days after apparent extinguishing
These fires are particularly dangerous because they cannot be smothered like traditional fires. The oxygen production makes standard fire suppression techniques ineffective.
Water cooling remains the primary method for controlling large lithium-ion battery fires, though specialised extinguishers exist for smaller incidents.
Incidence and Severity of Fires
Lithium-ion battery fires remain relatively rare but are increasing in frequency. Fire services report growing numbers of battery-related incidents as these batteries become more common in homes and workplaces.
Common locations for battery fires:
- Electric vehicles and charging stations
- Mobile phones and laptops
- E-bikes and scooters
- Power tools and equipment
The severity of these fires depends on the battery size and number of cells involved. Small device batteries may cause localised damage, whilst electric vehicle batteries can destroy entire structures.
Large battery arrays require significant water application for extended periods to achieve extinguishment. Emergency services often need to cool batteries for hours or days after the visible flames disappear.
Property damage from lithium-ion battery fires tends to be severe due to the intense heat and toxic smoke production. The fires spread rapidly and can cause structural damage beyond the immediate burn area.
Causes of Lithium Battery Fires
Lithium-ion battery fires typically start when batteries overheat and enter a dangerous state called thermal runaway. Physical damage, overcharging, and improper storage create the conditions that lead to these fires.
Thermal Runaway and Battery Chemistry
Thermal runaway occurs when a lithium-ion battery overheats rapidly and cannot cool down. The battery's internal temperature rises quickly, causing chemical reactions that produce more heat.
This process creates a dangerous cycle. Heat triggers more chemical reactions, which produce even more heat. The battery becomes extremely hot and can catch fire or explode.
During thermal runaway, the battery releases toxic gases. These gases are flammable and can ignite. The fire spreads quickly and burns very hot.
Lithium-ion batteries are extremely sensitive to high temperatures and inherently flammable. Once thermal runaway begins, it is very difficult to stop.
The electrolyte inside the battery breaks down when it gets too hot. This breakdown releases oxygen, which feeds the fire and makes it burn stronger.
Common Triggers: Overcharging, Damage, and Faults
Physical damage is one of the main causes of lithium-ion battery fires. Dropping, crushing, or puncturing a battery can damage the internal components. This damage creates short circuits that generate heat.
Overcharging happens when a battery receives too much electrical current. The extra energy turns into heat, which can start thermal runaway. Using the wrong charger increases this risk.
Manufacturing defects can cause batteries to fail unexpectedly. Poor quality control during production may leave contamination or design flaws inside the battery.
Common manufacturing problems include:
- Faulty separators between battery cells
- Metal contamination during assembly
- Poor welding of internal connections
- Inadequate quality testing
Age and wear make batteries more dangerous over time. Old batteries are more likely to develop internal faults. Their performance degrades, making them unstable.
Environmental and Storage Factors
Temperature plays a major role in battery safety. Extreme temperatures can increase the risk of short circuits or thermal runaway.
Hot environments stress the battery and can trigger overheating. Storing batteries in direct sunlight or hot cars creates dangerous conditions.
Cold temperatures can also cause problems. When cold batteries warm up quickly, condensation can form inside and cause electrical faults.
Poor ventilation prevents heat from escaping around batteries. This trapped heat builds up and increases fire risk.
Storage near metal objects creates hazards. Metal can cause short circuits if it touches battery terminals. Keys, coins, and tools should be kept away from loose batteries.
Humidity can cause corrosion of battery contacts. This corrosion creates resistance, which generates heat during use.
Proper storage requires cool, dry places away from flammable materials. Batteries should never be stored in extreme temperatures or cramped spaces without airflow.
Risks and Hazards Associated with Lithium Battery Fires
Lithium battery fires present unique dangers that differ significantly from conventional fires. These incidents involve intense heat, toxic gas emissions, and flames that resist traditional suppression methods.
Dangers of Fire and Explosion
Lithium-ion batteries pose significant fire hazards due to thermal runaway, a self-sustaining chemical reaction that rapidly increases temperature. This process creates intense fires that burn much hotter than ordinary household fires.
The batteries can explode without warning during thermal events. Battery fires can launch projectiles 30 to 40 feet from the initial location, creating additional hazards for people nearby.
Key explosion risks include:
- Sudden battery cell rupture
- Flying debris and hot materials
- Secondary fires from projectiles
- Rapid fire spread to nearby objects
These fires burn at temperatures exceeding 1,000°C. The extreme heat can quickly ignite surrounding materials and compromise building structures.
Battery fires also have the dangerous ability to reignite hours after appearing extinguished. This creates ongoing risks even after emergency services have responded to the initial incident.
Toxic Gas Emissions and Environmental Impact
Lithium battery fires emit toxic smoke containing harmful chemicals that pose serious health risks. The smoke contains hydrogen fluoride, carbon monoxide, and other poisonous gases.
Hydrogen fluoride is particularly dangerous as it can cause severe burns to skin and respiratory systems. Even brief exposure can lead to serious medical complications.
Toxic emissions include:
- Hydrogen fluoride
- Carbon monoxide
- Lithium compounds
- Electrolyte vapours
The toxic gases spread quickly through buildings via ventilation systems. Indoor fires become especially hazardous as the poisonous smoke concentrates in enclosed spaces.
Environmental contamination occurs when battery chemicals leak into soil and water systems. The toxic materials can persist in the environment for extended periods.
First responders and building occupants face immediate health risks from smoke inhalation. Proper evacuation and respiratory protection become critical during these incidents.
Challenges in Fire Suppression
Traditional fire suppression methods prove largely ineffective against lithium battery fires. Water can actually worsen the situation by spreading burning electrolyte materials.
Lithium-ion fires represent a chemical process rather than conventional combustion, making standard extinguishing techniques inadequate. The thermal runaway reaction continues internally even when external flames appear controlled.
Suppression challenges include:
- Water ineffectiveness
- Foam limitations
- Chemical suppression resistance
- Reignition potential
Fire services require specialised equipment and training for battery fire incidents. Standard fire-fighting procedures must be adapted to address the unique characteristics of these fires.
The fires often require complete battery submersion in water tanks for extended periods. This cooling method helps prevent reignition but demands significant resources and specialised facilities.
Multiple fire service visits may be necessary as batteries can reignite repeatedly. UK fire services now tackle at least three lithium-ion battery fires daily, highlighting the growing challenge for emergency responders.
Preventive Measures for Lithium Battery Fire Safety
Proper handling, charging protocols, and disposal methods significantly reduce lithium-ion battery fire risks. These measures focus on preventing thermal runaway and maintaining battery integrity throughout their lifecycle.
Best Practices for Safe Usage
Only use batteries from reputable manufacturers to ensure quality standards and safety certifications. Counterfeit batteries often lack proper safety mechanisms.
Physical Protection Guidelines:
- Protect batteries from physical damage, impacts, and punctures
- Avoid dropping devices containing lithium-ion batteries
- Never attempt to repair damaged battery casings
Temperature control remains critical for battery safety. Avoid storing batteries in extreme temperatures or direct sunlight. High heat can trigger thermal runaway.
Never leave devices in hot cars or near heating sources. Cold temperatures can also damage battery cells permanently.
Warning Signs to Monitor:
- Swelling or bulging battery cases
- Unusual heat generation during normal use
- Strange odours or hissing sounds
- Visible damage to battery casing
Stop using batteries immediately if any warning signs appear. Damaged batteries should never be recharged as this increases fire risk significantly.
Guidelines for Charging and Storage
Use only original chargers or genuine replacements for charging lithium-ion batteries. Incompatible chargers can cause overcharging and thermal runaway.
Safe Charging Practices:
- Never charge batteries unattended for extended periods
- Disconnect chargers once batteries reach full capacity
- Avoid overnight charging when possible
- Never cover chargers or charging devices
Charge batteries in well-ventilated areas away from escape routes. The London Fire Brigade reports that blocked escape routes contribute to lithium battery fire casualties.
Storage Requirements:Store batteries in cool, dry locations between 15-25°C when possible. Avoid basements, attics, or areas with temperature fluctuations.
Keep lithium-ion battery products separated during storage to prevent fire spread between devices. Store batteries away from flammable materials like paper, fabric, or chemicals.
For long-term storage, maintain batteries at approximately 50% charge. Fully depleted or fully charged batteries degrade faster during storage.
Safe Disposal and Recycling Protocols
Never dispose of lithium-ion batteries in regular household waste. Damaged batteries in waste trucks can cause fires during collection or processing.
Proper Disposal Locations:
- Local council recycling centres
- Electronics retailers with take-back programmes
- Specialised battery recycling facilities
- Automotive centres for electric vehicle batteries
Safely dispose of damaged batteries at recycling centres equipped for hazardous materials. These facilities have proper containment and fire suppression systems.
Transportation Safety: Transport damaged batteries in non-conductive containers. Avoid metal containers that could create short circuits.
Tape battery terminals with electrical tape to prevent accidental activation. Keep batteries upright during transport when possible.
For businesses disposing of large quantities, contact specialist waste management companies. They provide secure collection and certified destruction services.
Document battery disposal for compliance with environmental regulations. Many industries must track hazardous waste disposal for regulatory purposes.
Responding to Lithium Battery Fire Incidents
Effective response to lithium battery fires requires immediate evacuation, specialised suppression techniques, and coordinated emergency service actions. UK fire brigades now respond to more than three lithium-ion battery fires each day, making proper response protocols essential for public safety.
Initial Response Steps and Evacuation
The first priority during a lithium-ion battery fire is establishing safe evacuation distances. Emergency responders must create isolation zones to protect people from toxic gas exposure and potential explosions.
Immediate Actions Required:
- Evacuate the area immediately upon detecting battery fire signs
- Establish a minimum 30-metre perimeter around small batteries
- Expand isolation distances to 100+ metres for larger battery systems
- Position personnel upwind to avoid toxic gas inhalation
Battery fires produce dangerous gases including hydrogen fluoride and carbon monoxide. These gases are heavier than air and can accumulate in low areas.
Visual warning signs include swelling, unusual odours, smoke emission, or discolouration of battery casings. Personnel should never attempt to move damaged batteries without proper protective equipment.
Personal Protection Requirements:
- Self-contained breathing apparatus (SCBA)
- Electrical-rated protective gloves
- Full protective clothing against chemical exposure
Specialised Firefighting Methods
Traditional firefighting methods often prove inadequate for battery fires. Lithium-ion battery fires require specialised suppression techniques due to thermal runaway processes that can restart hours after initial suppression.
Water remains the most effective cooling agent, but massive quantities are needed. Fire services typically require thousands of litres to adequately cool battery systems and prevent reignition.
Effective Suppression Techniques:
- Apply large volumes of water for cooling rather than flame suppression
- Use Class D fire extinguishers for small battery fires
- Maintain continuous cooling even after flames appear extinguished
- Monitor temperatures for 24-48 hours post-incident
The main challenge is preventing thermal runaway propagation between battery cells. This self-sustaining chemical reaction generates extreme heat that traditional suppression cannot easily control.
Reignition poses significant risks. Batteries may restart thermal runaway days after initial suppression, requiring extended monitoring and cooling efforts.
Role of Emergency Services
Emergency services coordinate multi-agency responses involving fire brigades, police, and hazardous materials teams. Fire safety protocols for lithium batteries require specialised training and equipment beyond standard firefighting capabilities.
Fire services lead suppression efforts whilst police manage evacuation zones and traffic control. Hazmat teams assess toxic gas dispersion and environmental contamination risks.
Key Service Responsibilities:
- Fire Brigade: Primary suppression, cooling operations, technical rescue
- Police: Evacuation coordination, perimeter security, traffic management
- Hazmat Teams: Gas monitoring, environmental protection, waste disposal
Communication between agencies proves critical for effective response. Battery incidents often require manufacturer consultation for specific system shutdown procedures.
Emergency services must prepare for extended operations. Large battery systems can burn for days, requiring sustained resource deployment and personnel rotation.
Specialist equipment needs include thermal imaging cameras, gas detection meters, and high-volume water delivery systems specifically configured for battery fire scenarios.
Frequently Asked Questions
Lithium-ion battery fires occur primarily due to thermal runaway caused by overcharging, physical damage, or extreme temperatures. These fires require specific extinguishing methods and safety protocols that differ significantly from conventional fires.
What are the primary causes of fires in lithium-ion batteries?
Lithium-ion battery fires most commonly occur when batteries are damaged, overcharged, completely drained, or exposed to extreme temperatures. Physical damage such as punctures or crushing can create internal short circuits.
Overcharging represents one of the most frequent causes. This happens when batteries receive more power than they can safely handle.
Extreme heat or cold environments stress the battery cells. Manufacturing defects can also create weak points that lead to failure.
Internal short circuits trigger thermal runaway. This process generates temperatures exceeding 1,000°F and spreads rapidly between cells.
How can one safely extinguish a lithium battery fire?
Water-based extinguishers are needed to extinguish battery fires. Traditional fire extinguishers prove ineffective against lithium-ion battery blazes.
Large amounts of water delivered over significant time periods are required for electric vehicle battery arrays. The cooling effect of water helps prevent thermal runaway from spreading.
Never attempt to extinguish large battery fires without professional help. Contact the fire service immediately for any battery fire involving electric vehicles or energy storage systems.
Small device fires may be manageable with copious amounts of water. However, evacuation remains the safest option in most situations.
What safety precautions should be taken when handling lithium-ion batteries to prevent fires?
Choose products that are safety certified by recognised testing laboratories. Many online products lack proper safety standards and certifications.
Use only the charging equipment provided by the manufacturer. Third-party chargers may not match the battery's specifications correctly.
Store batteries away from extreme temperatures and direct sunlight. Keep them clear of exits and flammable materials at all times.
Never modify batteries or chargers in any way. Charge larger devices like e-bikes outside the home and never in escape routes.
Inspect battery-powered devices regularly for swelling, punctures, or other damage. Stop using devices immediately if any warning signs appear.
In what way do lithium-ion battery fires differ from other types of fires?
Thermal runaway generates incredible heat exceeding 1,000°F and produces toxic gases. This process can spread rapidly from cell to cell within the battery pack.
These fires burn extremely hot and release dangerous fumes. The gases produced are both flammable and toxic to humans.
Battery fires can progress in less than a minute from the first warning signs. Traditional fire suppression methods often prove inadequate against these blazes.
The fires may reignite even after appearing extinguished. Thermal runaway can continue internally despite external cooling efforts.
Water becomes the primary suppression method rather than foam or chemical agents. Large volumes are needed to cool the batteries effectively.
What steps should be followed in the event of a lithium battery fire emergency?
Evacuate immediately upon noticing warning signs such as hissing sounds or unusual odours. White or grey wispy smoke indicates immediate danger of thermal runaway.
Follow established fire escape plans without delay. Call emergency services once safely outside the building.
Never attempt to move burning battery-powered devices. The risk of explosion and toxic gas exposure is too great.
Inform firefighters about the battery fire specifically. This helps them prepare appropriate equipment and safety measures.
Remain outside until emergency services declare the area safe. Battery fires can reignite unexpectedly hours later.
How can the risk of a lithium battery fire be minimised during the charging process?
Never charge devices overnight or whilst sleeping. Unattended charging prevents quick response to emerging problems.
Charge devices on hard, non-flammable surfaces away from beds and furniture. Avoid charging on carpets, sofas, or other soft materials.
Monitor devices during charging for excessive heat or swelling. Disconnect immediately if the battery becomes unusually warm.
Keep charging areas well-ventilated and accessible. Ensure charging locations remain clear of clutter and escape routes.
Never charge damaged batteries or devices showing wear. Replace batteries exhibiting any signs of deterioration immediately.
Unplug chargers when not in use to prevent electrical faults. Store charging cables properly to avoid damage that could cause problems.




%201.png)

%201.svg)